Hydrogen Development to Improve HEM Fuel Cells
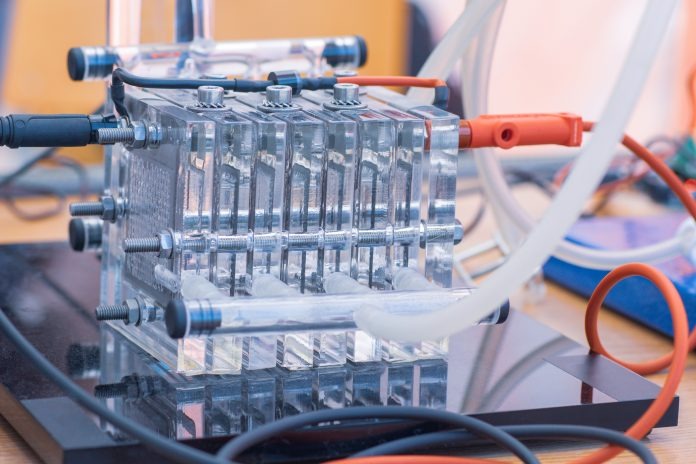
In a first-of-a-kind breakthrough, Hydrogen Industry Leaders highlights researchers use of hydrogen fuel cells for carbon capture, bringing environmentally friendly fuel cells to transportation and technology.
Using an electrochemical system powered by hydrogen, the technology can capture 99% of carbon dioxide. To do this, it converts fuel chemical energy directly into electricity.
These fuel cells can be used in transportation for zero-emission vehicles by controlling the short-circuited fuel cell through hydrogen.
Potentially, it can change the landscape of the development of hydrogen in the transport sector.
Engineers from the University of Delaware have been improving hydroxide exchange membrane (HEM) fuel cells within the research. Providing an economical and environmentally friendly alternative to traditional acid-based fuel cells is at the heart of the research.
The shortcomings of HEM fuel cells have been widely reported, so the possibility of building upon these for cleaner uses are positive. This was addressed in the Nature Energy journal, which can be read here.
Extensive research is underway to improve HEM fuel cells
Upon further research, they found that As HEM fuel cells are extremely sensitive to carbon dioxide in the air, the carbon dioxide makes it hard for a HEM fuel cell to breathe. The defect reduces the performance and efficiency by up to 20%.
With the defect in mind, it makes the fuel cell no better for the environment than conventional combustion engines. Turning problems into solutions with this improved fuel cell, the researchers hope to establish this technology to stimulate more carbon dioxide removal.
Part of the way they did this is by building a “self-purging” process in a separate device upstream from the fuel cell stack, so they could later turn it into a carbon dioxide separator.
Yan, Henry Belin du Pont Chair of Chemical and Biomolecular Engineering, said: “It turns out our approach is very effective. We can capture 99% of the carbon dioxide out of the air in one pass if we have the right design and right configuration.”
Brian Setzler, assistant professor for research in chemical and biomolecular engineering and paper co-author, added: “Once we dug into the mechanism, we realized the fuel cells were capturing just about every bit of carbon dioxide that came into them, and they were good at separating it to the other side.”
How exactly does it work?
The team then constructed a compact, spiral module with a large surface area in a small volume – essentially making a smaller package capable of filtering greater quantities of air at a time, making it both effective and cost-effective for fuel cell applications.
An early prototype spiral device about the size of a 12-ounce soda can already filter 10 litres of air per minute and scrubbing out 98% of the carbon dioxide. Roughly the size of a gallon of milk, the device could be used to remove carbon dioxide from many places.
From their research, they found that an electrochemical cell measuring 2 inches by 2 inches could always remove about 99% of the carbon dioxide found in the air flowing at a rate of approximately two litres per

